SMS 2023 Symposium
Image & Video Competition
Thank you to Nikon for sponsoring the Image competition. The winning entry will get £50.
Competition Entries
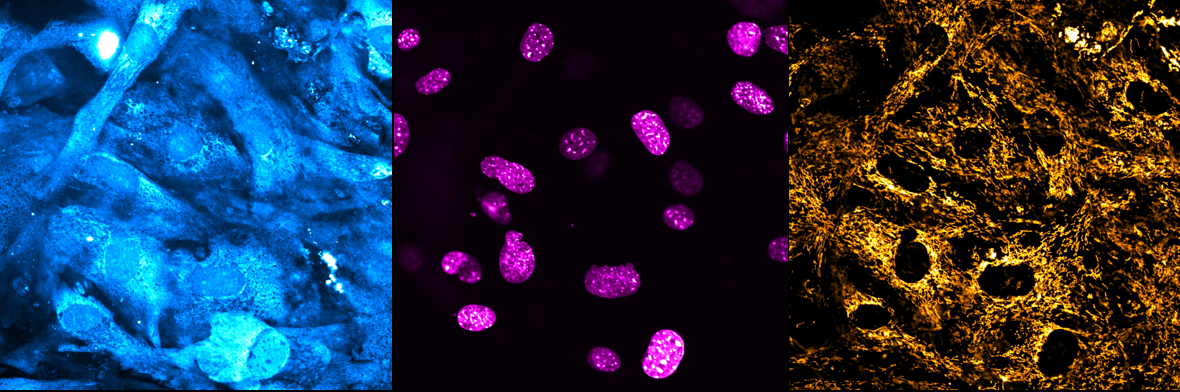
1. Illuminating the role of mitochondrial elongation in vascular calcification
Augustina Salis, University of Edinburgh
Our research project focuses on elucidating the impact of mitochondrial elongation in vascular calcification. We conducted imaging studies employing spinning-disk confocal microscopy (Opera Phenix Plus High-Content Screening System) in the High Content Screening Facility to comprehensively assess whether mitochondria undergo elongation in vascular smooth muscle cells (VSMCs) cultured under calcifying conditions to model vascular calcification. The three channels on the left, middle, and right represent stains for calcium, the nucleus, and mitochondria, respectively. Leveraging machine learning techniques and segmentation protocols, our objective is to analyse whether mitochondria situated in close proximity to calcium deposits (seen as bright structures in the calcium channel) exhibit greater elongation and adopt a more extensive network-like structure in comparison to those distanced from calcium deposits.
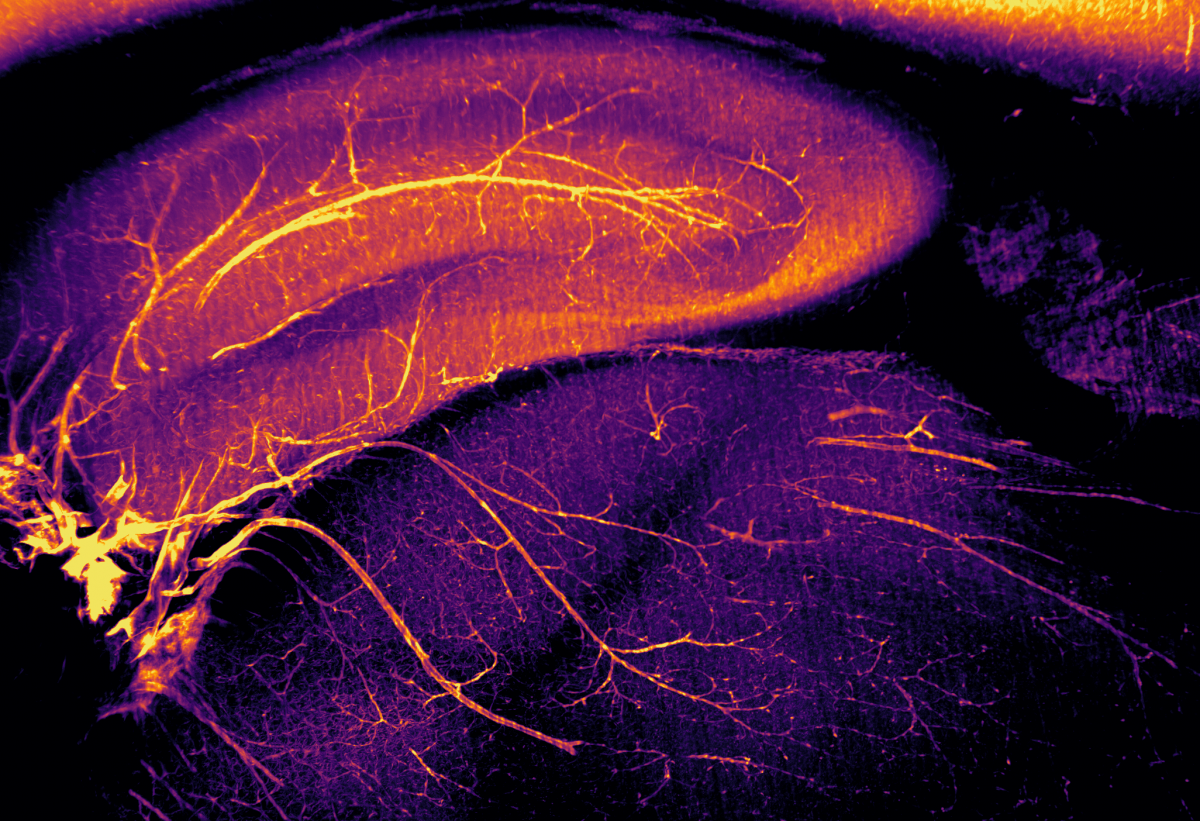
2. Squid
Dr Cristina Martinez-Gonzalez, University of Edinburgh
Light sheet microscopy image showing the vasculature of the hippocampus and thalamus in a rat brain. The entire rat brain was optically cleared using our RatDISCO protocol and blood vessels were visualised using the biotinylated lectin IsoB4. Images were acquired in the sagittal plane using an Ultramicroscope II (maximum intensity projection; single field of view, final magnification 3.5x) with an Olympus MVPLAPO 2x objective, working distance 6 mm; Andor Neo sCMOS camera and a 561nm laser. The image was adjusted in FIJI for brightness and contrast and assigned an inferno LUT. Imaging the whole brain without mechanical sectioning allowed us to rapidly visualize the intact vasculature of the brain. Light sheet microscopy provides comprehensive, unbiased (and beautiful) information that traditional sectioning methods cannot achieve.
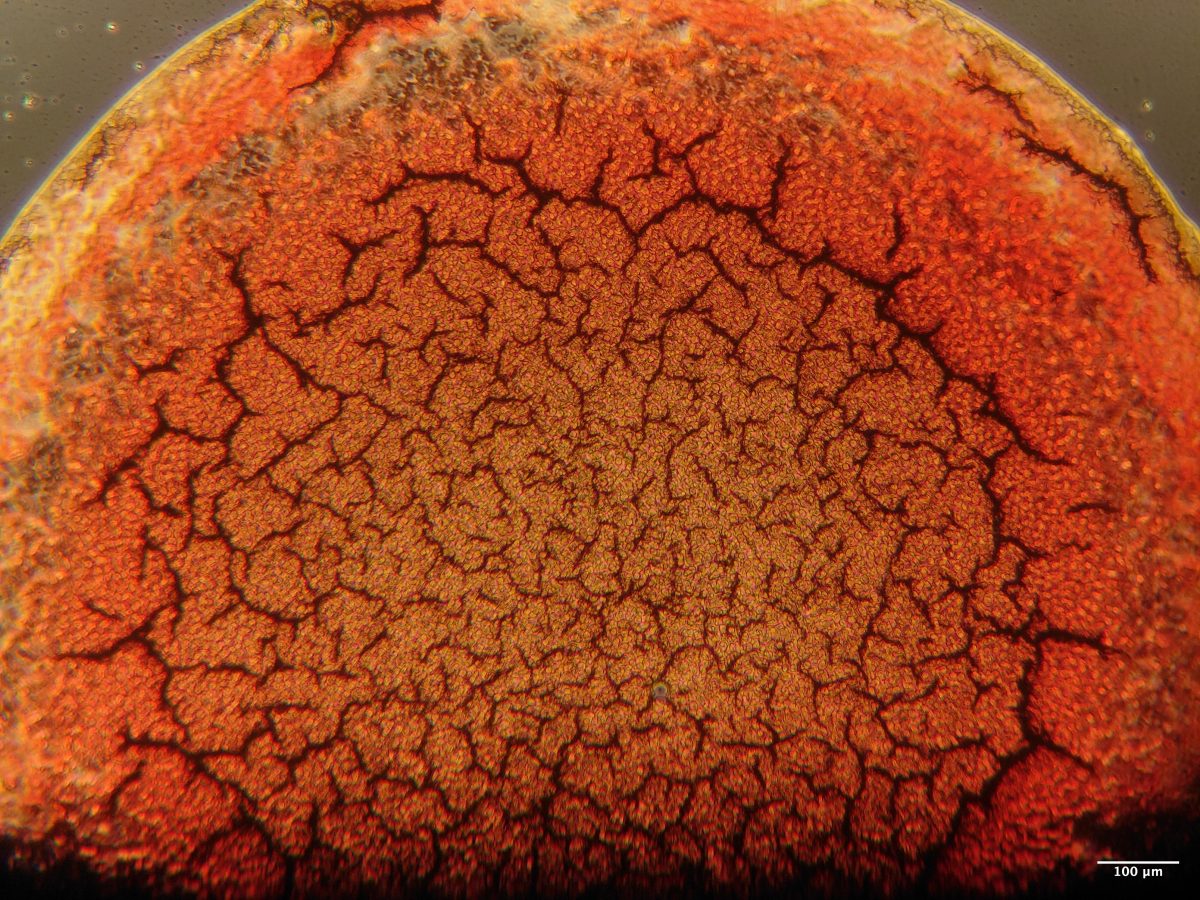
3. Carpe Jugulum
Jessany Marsden, University of Edinburgh
Human Blood drop taken using a ZEISS Primovert microscope with a 4X objective lens. My project looks to underpin applied forensic science with soft matter physics. This image is of my own, untreated blood taken the same day of imaging. Dried blood droplets often show interesting drying patterns, which can potentially be used to infer information about the source of the blood. This droplet shows radial cracks which increase in size from the centre, with individual red blood cells visible in between.
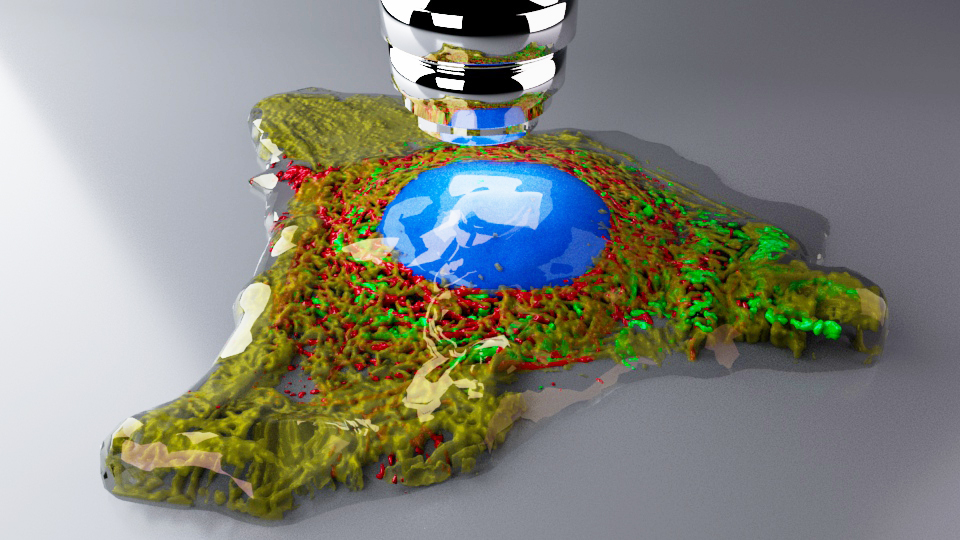
4. A COS cell's digital twin
Prof Craig Daly, University of Glasgow
A single COS cell was stained and scanned on a confocal microscope (40x objective, NA 1.4). Stains for nucleus (blue), mitochondria (green), actin (red) and tubulin (yellow). Z-series were segmented using Slicer3D and exported to Autodesk Maya. The mesh (.obj) files for each channel were retopologised to repair holes and any non-manifold geometry. Arnold renderer was used to create the final photorealistic shot using 4 area lights. The objective was added for fun/context. More importantly, the data is now in a format that can be used for animations and/or VR and AR applications. Volumes and surface area values can also be obtained from the ‘digital twin’.
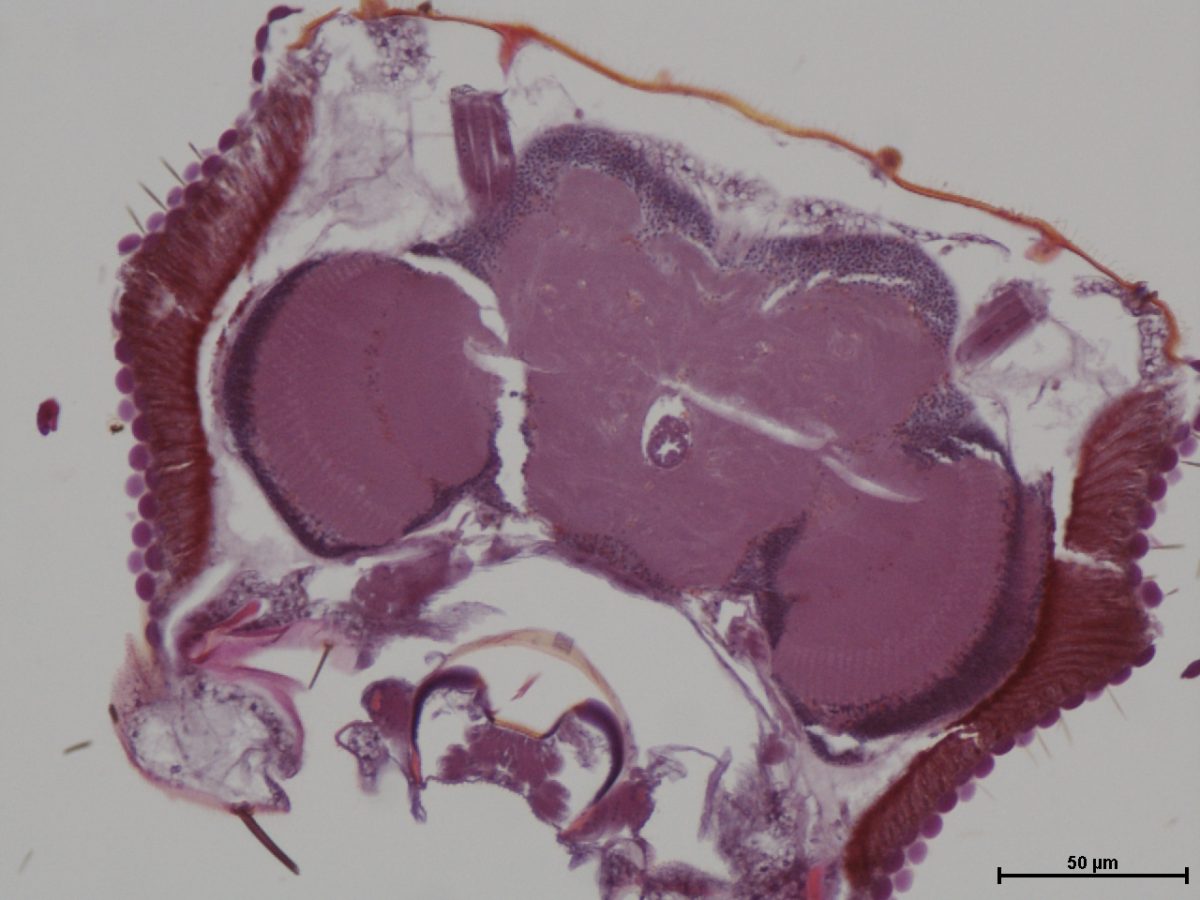
5. Brain of male old-adult (28-day-old) Drosophila melanogaster fly. (H&E stain; transverse section at level of mid-brain, x200). Bar, 50 µm.
Fred Ssempijja, University of Edinburgh
This image depicts a transverse section of the brain of a male old-adult (28-day-old) Drosophila melanogaster fly, at level of mid-brain, stained using H&E stain. It was taken during a study using Drosophila as a model for neurological disorders of a human disease (epilepsy).
Histological procedures and examination of brain tissues of the flies were performed at the Central diagnostic Laboratory at the College of Veterinary Medicine Animal Resources and Bio-security, Makerere University.
6. Flaming worms
Ritika Siddiqui, University of Dundee
The worms shown in the video are C. elegans isolated from the wild. These worms are made to express mCherry tagged protein through their body. Such tagging of proteins with fluorescent marker allow us to identify the recombinant progeny coming from mating these worms with other genotypes of worms.
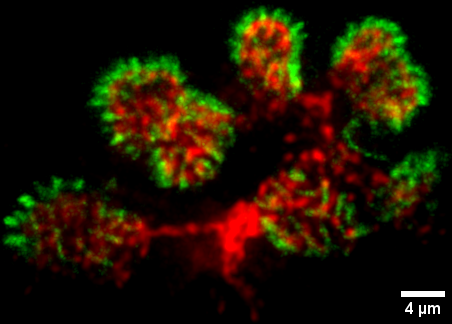
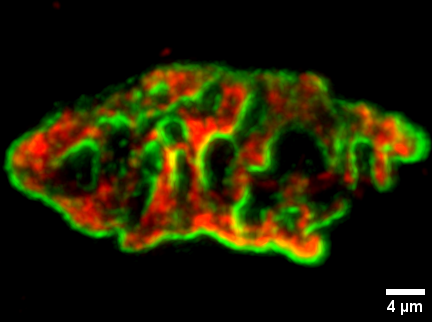
8. Expansion Microscopy (4x) of Neuromuscular Junctions (Mouse)
Abdullah Ramadan, University of Edinburgh
The neuromuscular junction (NMJ) bridges lower motor neurons and muscle fibers. It’s a valuable model for studying synapses due to its accessibility and simplicity. Its primary components are nerve terminals and Acetylcholine receptors (AChRs). NMJ abnormalities are linked to neuromuscular diseases.
Human and mouse NMJs differ in shape, with human NMJs being disc-shaped, while mouse NMJs have a pretzel-like structure. Super-resolution microscopy (Electron Microscope, 3D SIM, and d-STORM) reveals striped AChRs patterns in mouse NMJs and consistent distributed SNAP25 labeling. In contrast, human NMJs show localized fluorescence hotspots in motor nerve terminals.
In this study, we applied Expansion Microscopy (ExM) using an Acrylamide/Na+ Acrylate monomer solution protocol. Our results demonstrate the feasibility of 4x ExM for studying NMJs. We confirmed similar observations between human and mouse NMJs, previously detected by super-resolution microscopy, which confocal microscopy alone cannot capture.
Image details: α-Bungarotoxin Alexa Fluor 488 (Green) labels AChRs, while Mouse anti-SV2 IgG Alexa Fluor 594 (Red) labels synaptic vesicles. Images were captured using Nikon A1R FLIM confocal microscopy with specific settings: 20X objective lens, 512-pixel frame size, 0.5 mm Z-stack intervals, and 9x zoom. Huygens deconvolution software was applied. Scale bar =4 µm (with expansion factor of 4x ≈1 µm).
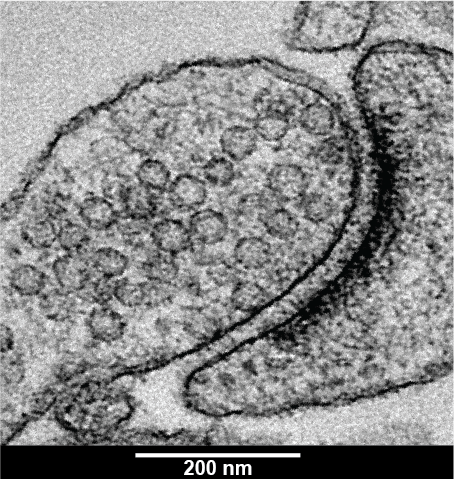
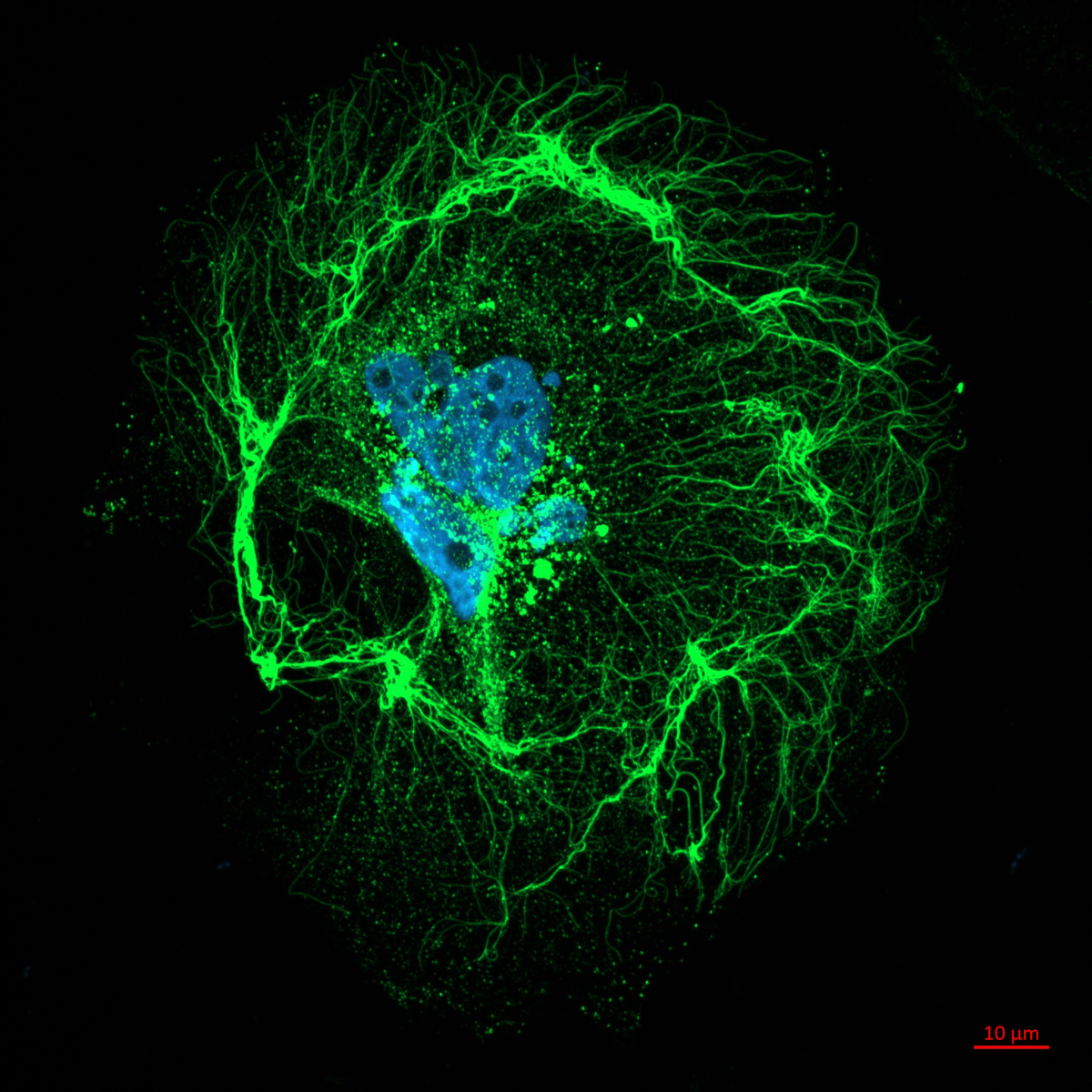
9. Beyond the surface: a glimpse into the structure of a synapse
Akanksha Jain, University of Edinburgh
Image shows the ultrastructure of a synapse of a hippocampal neuron taken from a mouse brain. At a synapse, neurons communicate through fast electrical and chemical signalling. The synapse is made of a pre-synapse (part found at the end of a neuron) containing several spherical structures called synaptic vesicles (SVs). These contain neurotransmitters, which are chemicals crucial for transmitting electrical signals from one neuron to another. These chemicals release across the gap between the neurons when SVs fuse with the membrane of the pre-synapse. Neurotransmitters induce electrical signal in the post-synapse (part of another neuron) opposite of the pre-synapse, seen in the image as a very dense dark region. Neuron communication is a very fast event and SVs are very small (~40 nm), making it a challenge to observe how SVs behave during these events at small synapses. With Zap and Freeze EM technique used here, electricity is passed through the neurons which triggers neuronal activity similar to how it happens in our brain. Neurons are quickly frozen within 5 ms using high-pressure freezing capturing snapshots of synapse frozen in time. These are imaged at a high magnification with transmission electron microscope, providing resolution to less than 200 nm.
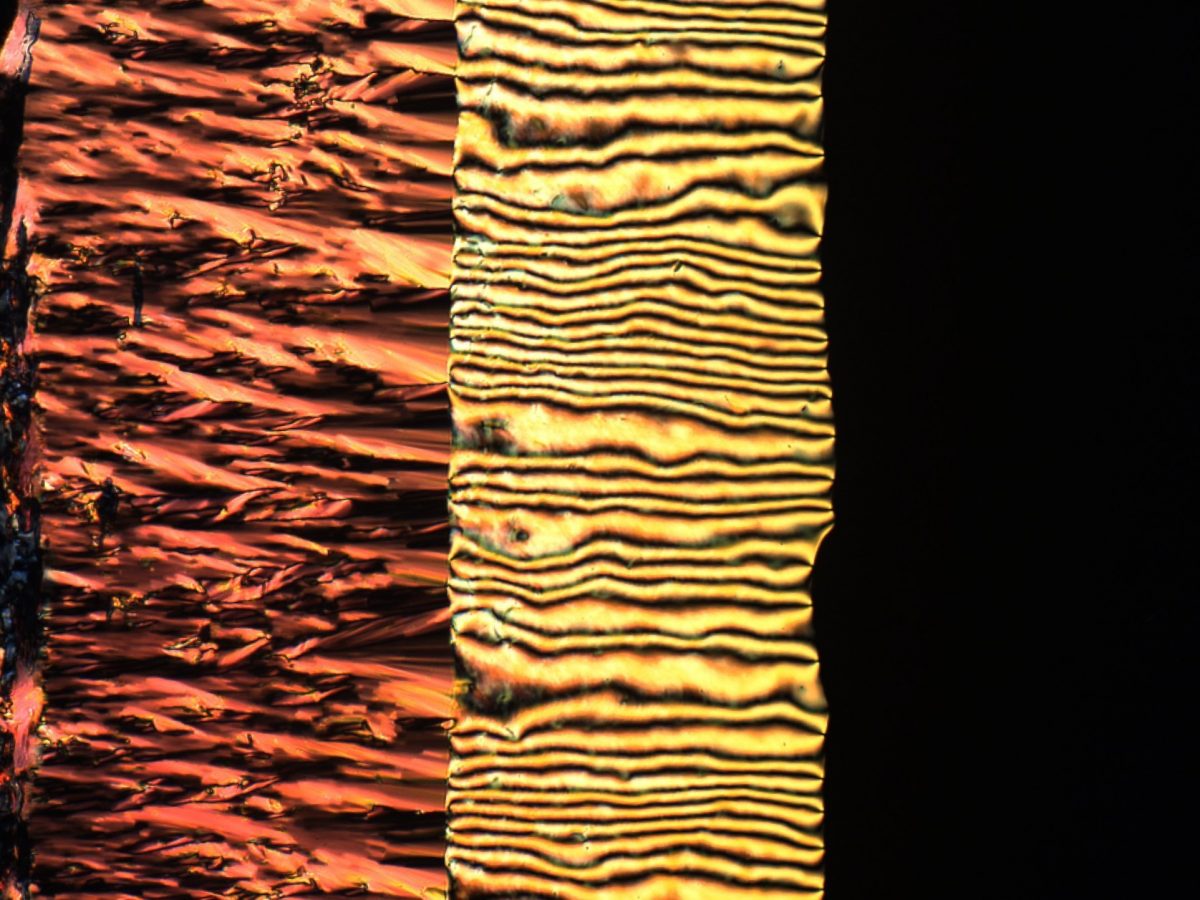
10. Liquid crystal phase transition
Órlaith Skelton, University of Edinburgh
A lyotropic chromonic liquid crystal, sunset yellow (SSY), is imaged between crossed polarisers at 10x magnification. Light patterns are made visible via the birefringence of the sample due to its anisotropy. In this one image we can clearly see three different mesophases occur. At high concentrations (left) is the highly ordered columnar phase. As water has evaporated at the edge of the sample, the concentration of SSY has greatly increased causing a highly ordered state to occur. At intermediate concentrations (centre) we can see the partially ordered nematic phase. Here the sample has some orientational order but no positional order, creating the ‘Schlieren’ texture. At low concentrations (right) the sample is no longer ordered, thus no longer birefringent, and so it appears dark due to the crossed polarisers.
11. Saos-2 stimulated to produce collagen extracellular matrix
Charlotte Clews, The Roslin Institute
Human osteoblast-like cell line Saos-2 stained for Collagen I using indirect immunofluorescence (Collagen I antibody, Alexafluor488) and DAPI (405), captured on a Zeiss 800 confocal microscope using 63x oil immersion lens. Large collagen bodies, smaller vesicles, and long fibrils can be seen, highlighting the different stages of collagen production, trafficking and function in the bone cell niche.
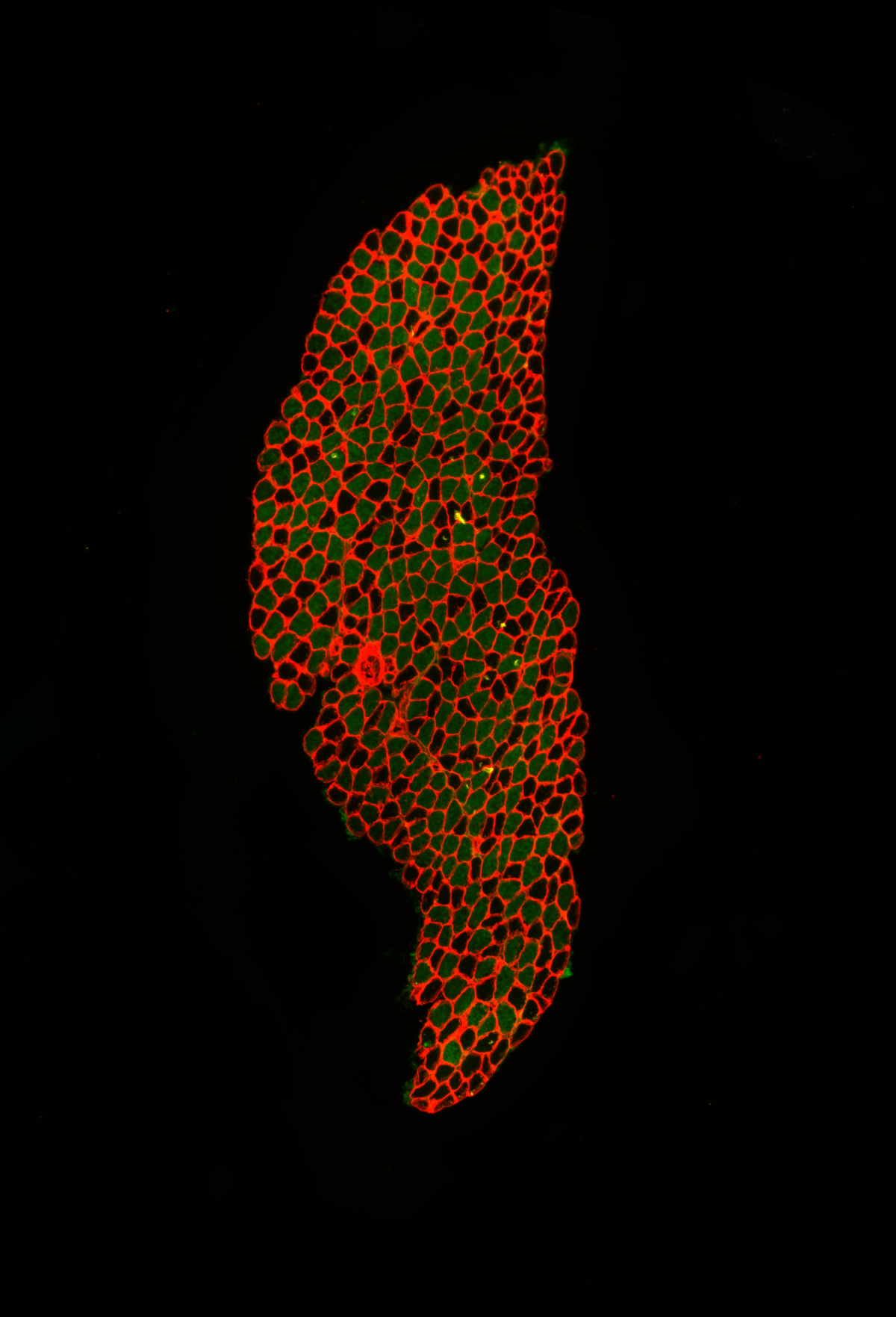
12. Fibre typing of Mouse Soleus Muscle
Rizwan Farrukh, The University of Edinburgh, Gillingwater Lab
Fibre typing of mouse skeletal muscle. x20 epifluorescence image of soleus muscle dissected from a 19-days-old mouse. 10um skeletal muscle cross section is stained with anti-Laminin 1+2 and BA-D5 antibodies to label skeletal muscle laminin (red) and myosin heavy chain Type I (green), respectively. Fibre typing is performed to study fibre type switching of the skeletal muscle from fast to slow as a result of disease process.
Get in touch with the Scottish Microscopy Society!
Feel free to contact us or join our mailing list using the contact form below.