Winner of the 2021 'Images of Research' Image Competition, sponsored by Horiba
Winner of the 2021 'Judges Choice' Image Competition, sponsored by FocalPlane
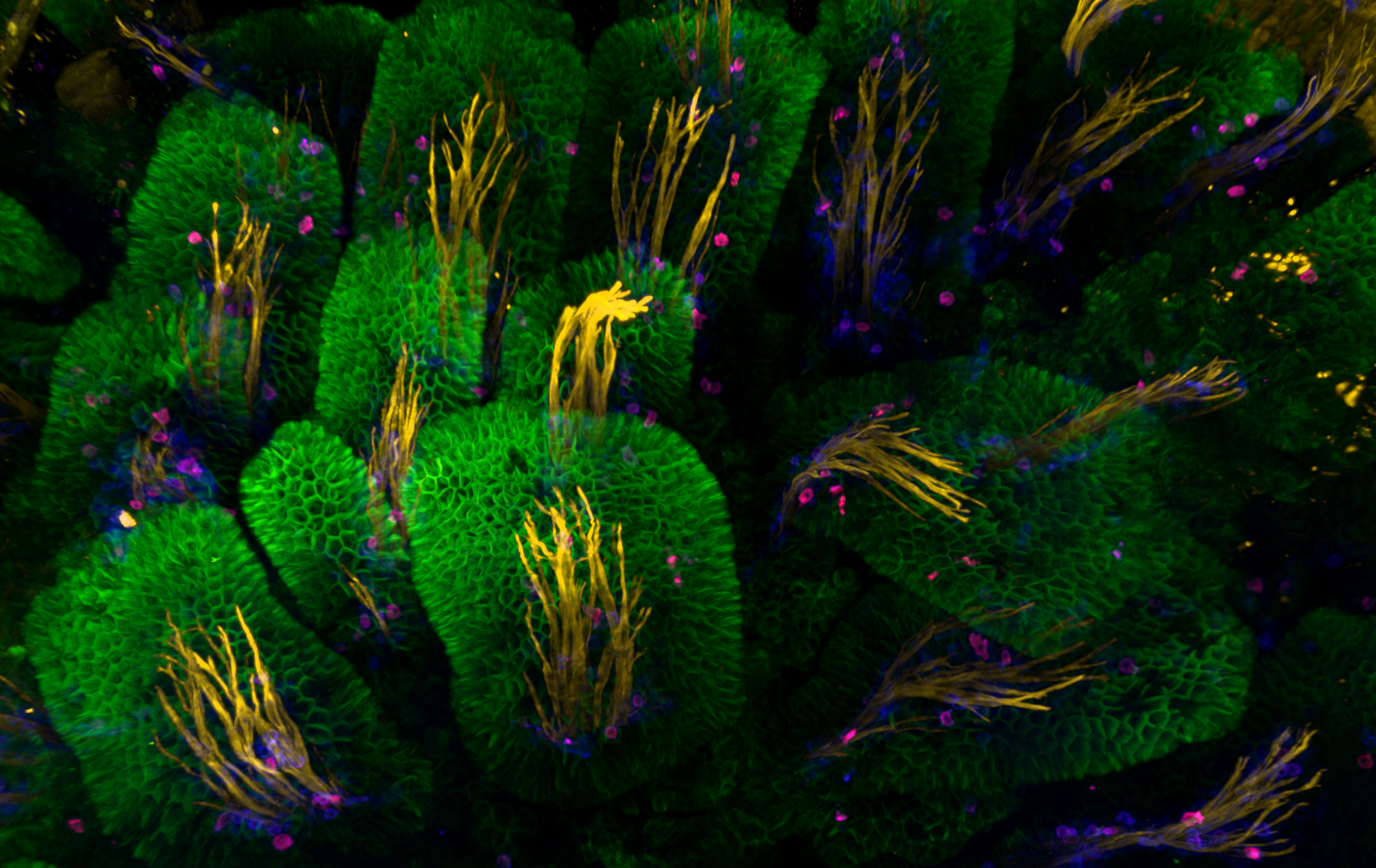
Intestial Forest
Jamie Whitelaw, CRUK Beatson Institute
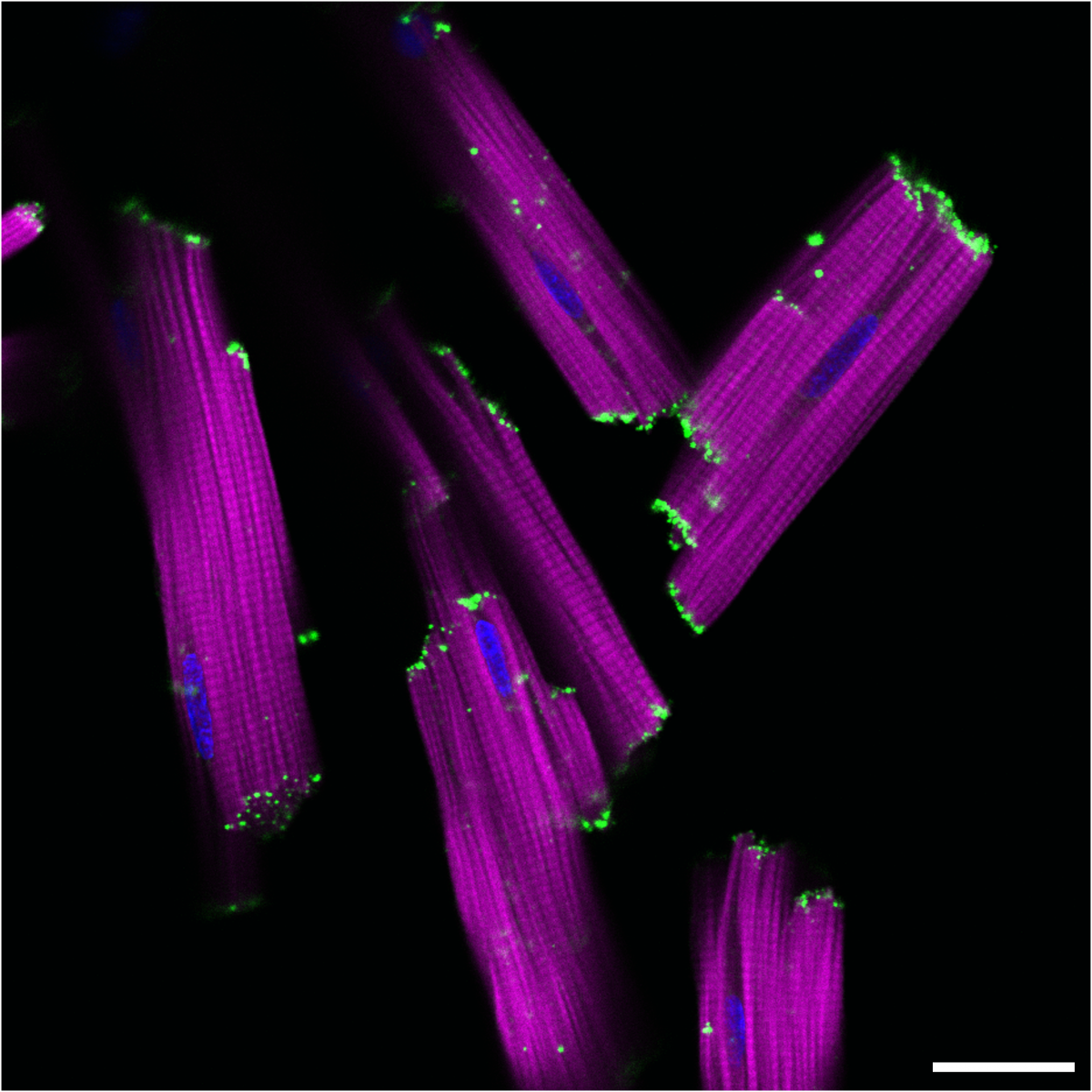
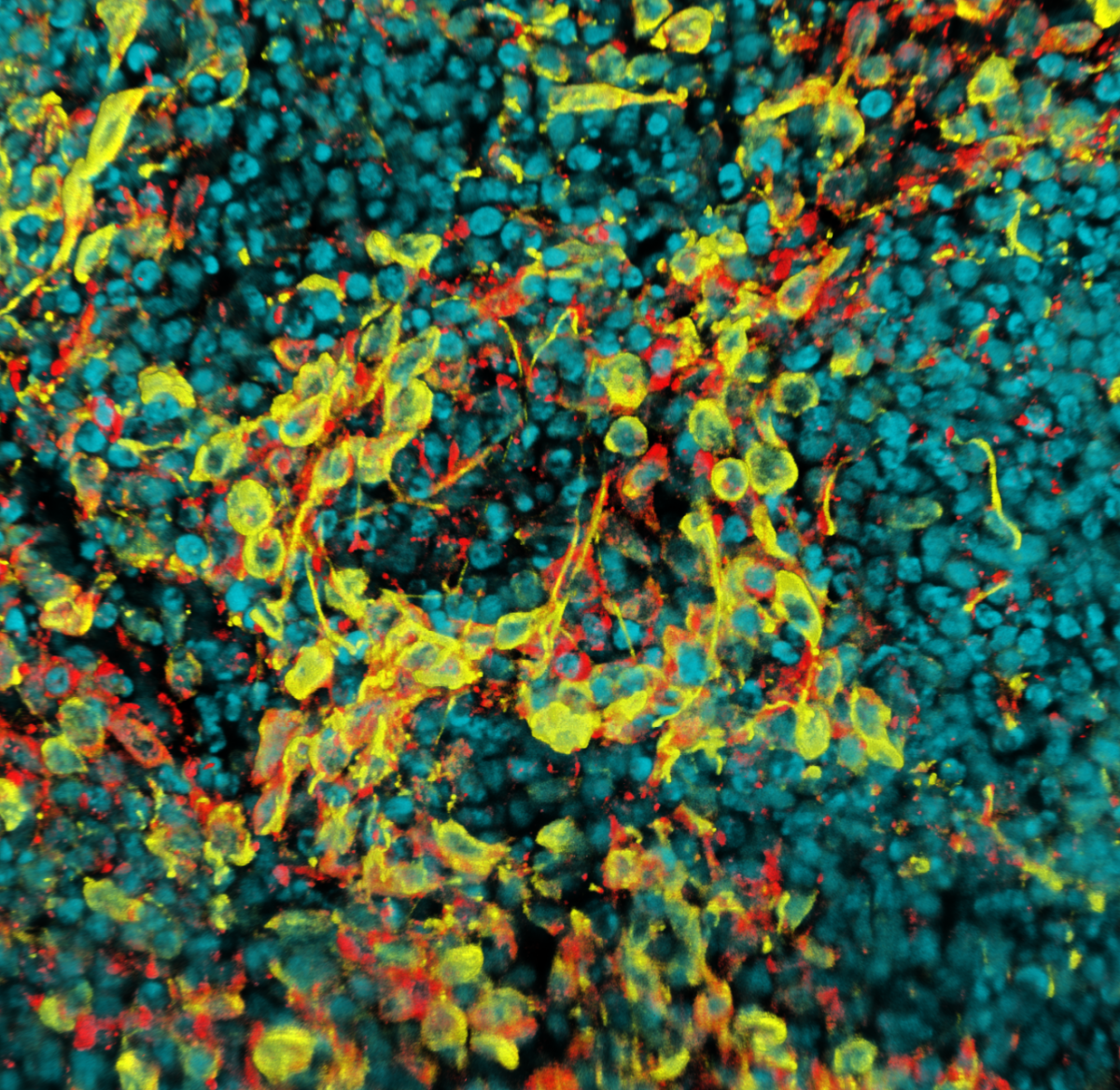
NckAP1 KO B16-F1 cells migrating through matrigel in a circular invasion assay. Cells were fixed and stained using Phalloidin in magenta, Tubulin in cyan and DAPI in yellow. Z-stacked, tile scan images were taken on the Zeiss 880 AiryScan at 20X objective and displayed at a max projection. Note that for some unknown reason, the tubulin staining appears reduced in follower cells compared to the leader cells, while F-actin remains uniform throughout.
Competition Entries
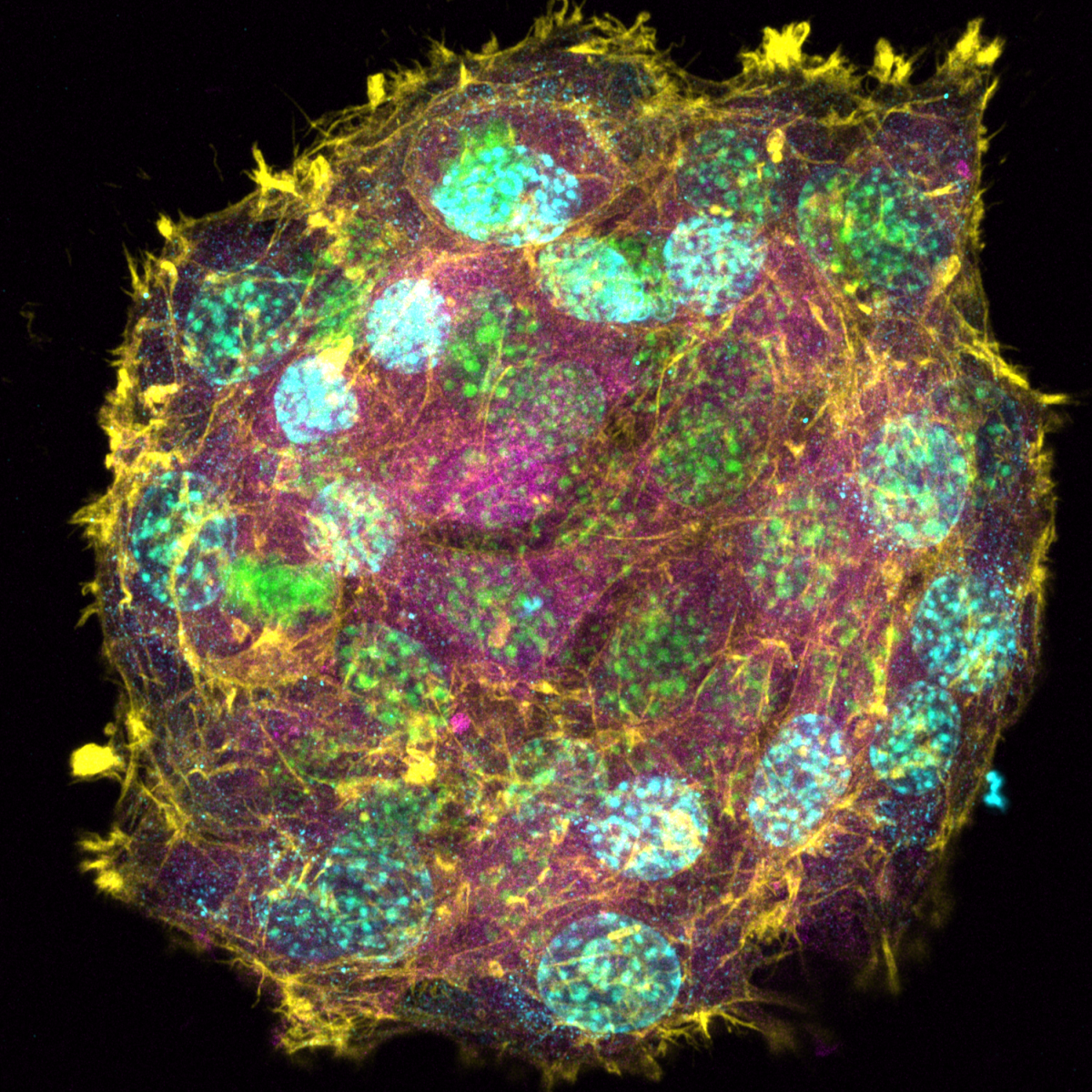
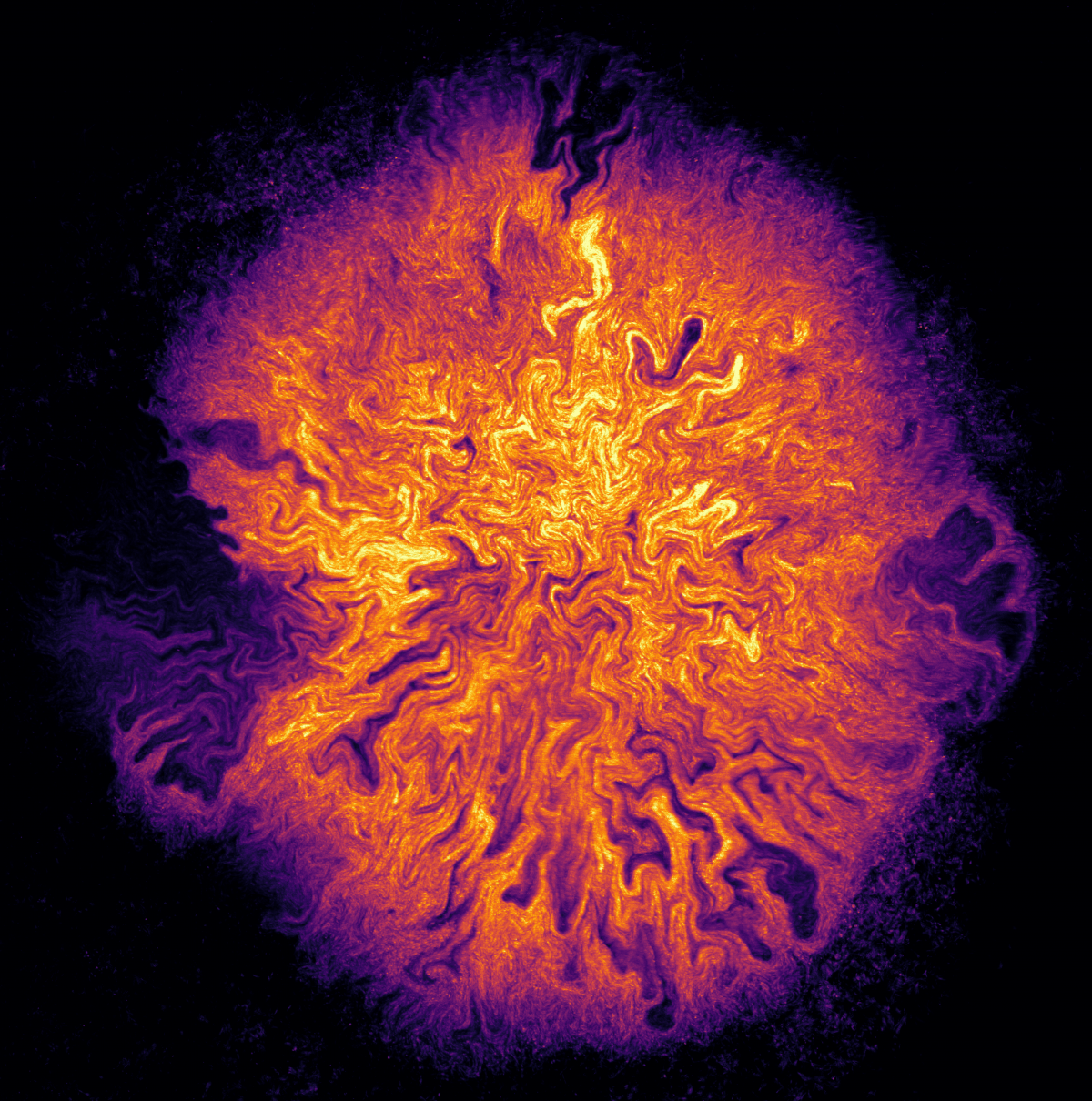
1. Oil Spill Galaxy
Tony Gutierrez, Heriot Watt University
Associate professor of Environmental Microbiology & Biotechnology
The photo was taken under a light microscope with angled light showing a marine oil snow (MOS) aggregation composed of marine exopolymeric substances (fluffy blue-white cloud at centre of image) with embedded oil droplets (shiny brown spheres) and that are also dispersed in the surrounding seawater. MOS was formed in unprecedented quantities during the Deepwater Horizon oil spill in the Gulf of Mexico.
2. Abstract art, or aspiring architecture?
Charlotte Clews, The Roslin Institute
PhD Student
The image shows a particularly picturesque example of collagen deposition, the initial “architectural” stage of hard tissue formation in bone through the production of collagen by osteoblasts before hydroxyapatite mineralisation. The image was acquired while undergoing an MRes investigating the molecular basis of a rare form of Osteogenesis Imperfecta in children which causes defects in collagen deposition and subsequent bone density, of which the cause is unknown.
Represented in the image are two complete stages of collagen production and deposition: firstly the vesicular collagen in the centre of the image, after undergoing ER-Golgi production and trafficking within the osteoblast, is released into the extracellular matrix. We can then observe the successful deposition of this collagen into the 20 – 300 nm diameter fibrils which arch around the cell peripheries. These structures form the collagen scaffold primed for calcium phosphate mineralisation, with this particular image showing the intricacy and artistry of this bio-synthetic process.
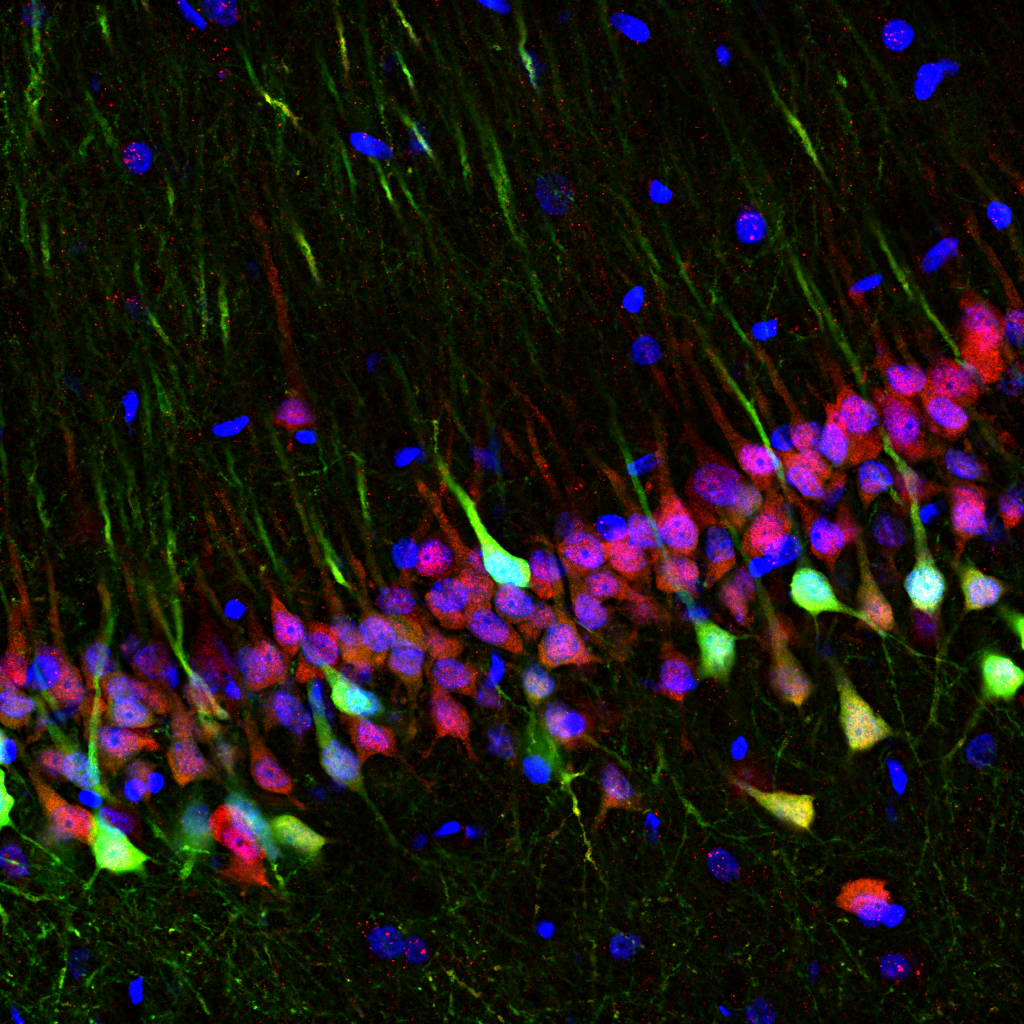
3. Neurons at sea
Francesco Gobbo, University of Edinburgh
Postdoctoral Researcher
The hippocampus is one of the main brain regions involved in space and memory processing. Here, pyramidal neurons are genetically engineered to express an optical calcium sensor to image their activity in real time with special miniature microscopes.
In this image, the green channel is the endogenous fluorescence of the GCaMP calcium sensor, the red one the anti-NeuN staining visualised with anti-guinea pig Alexa 647 secondary antibody, and in blue is the DAPI staining of cell nuclei.
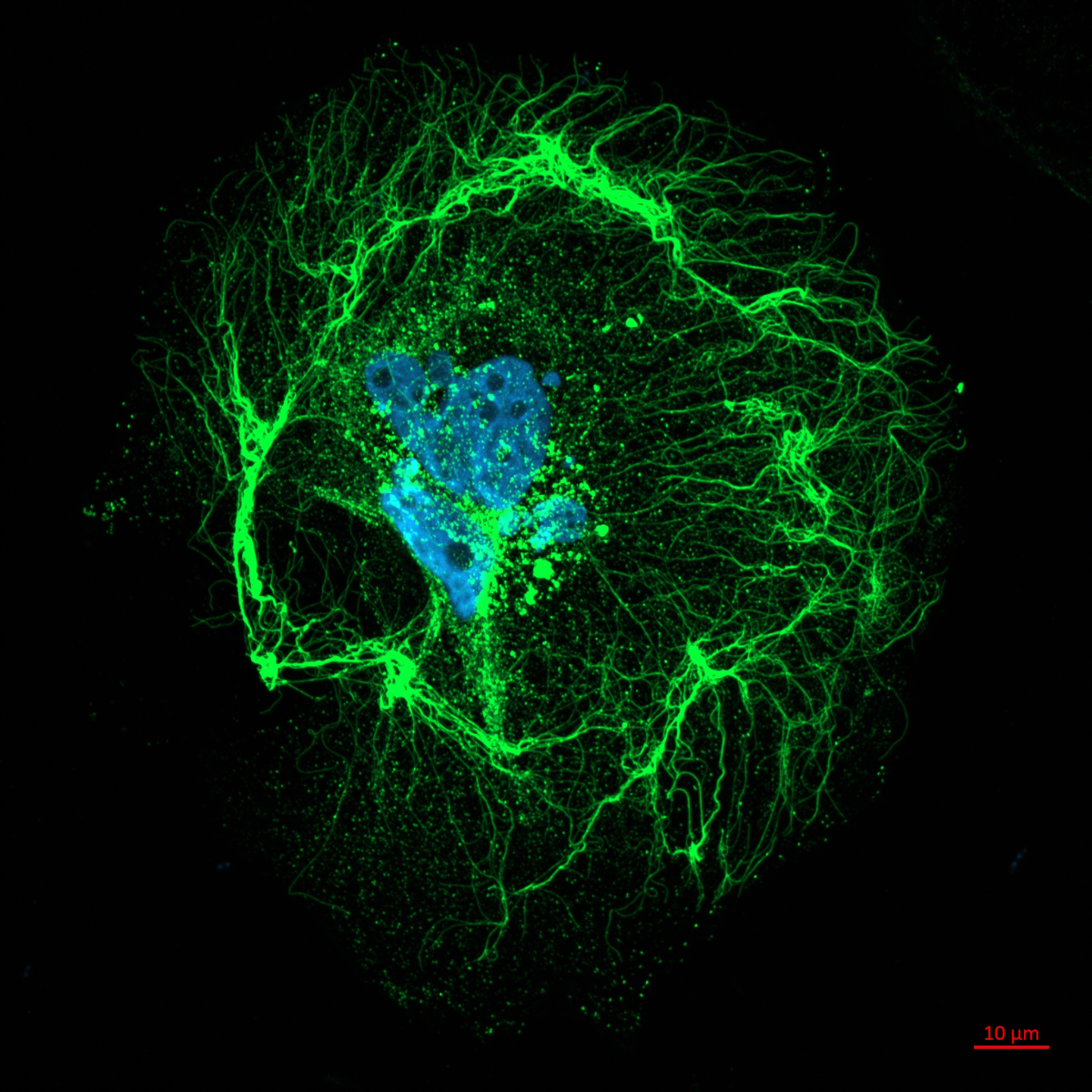
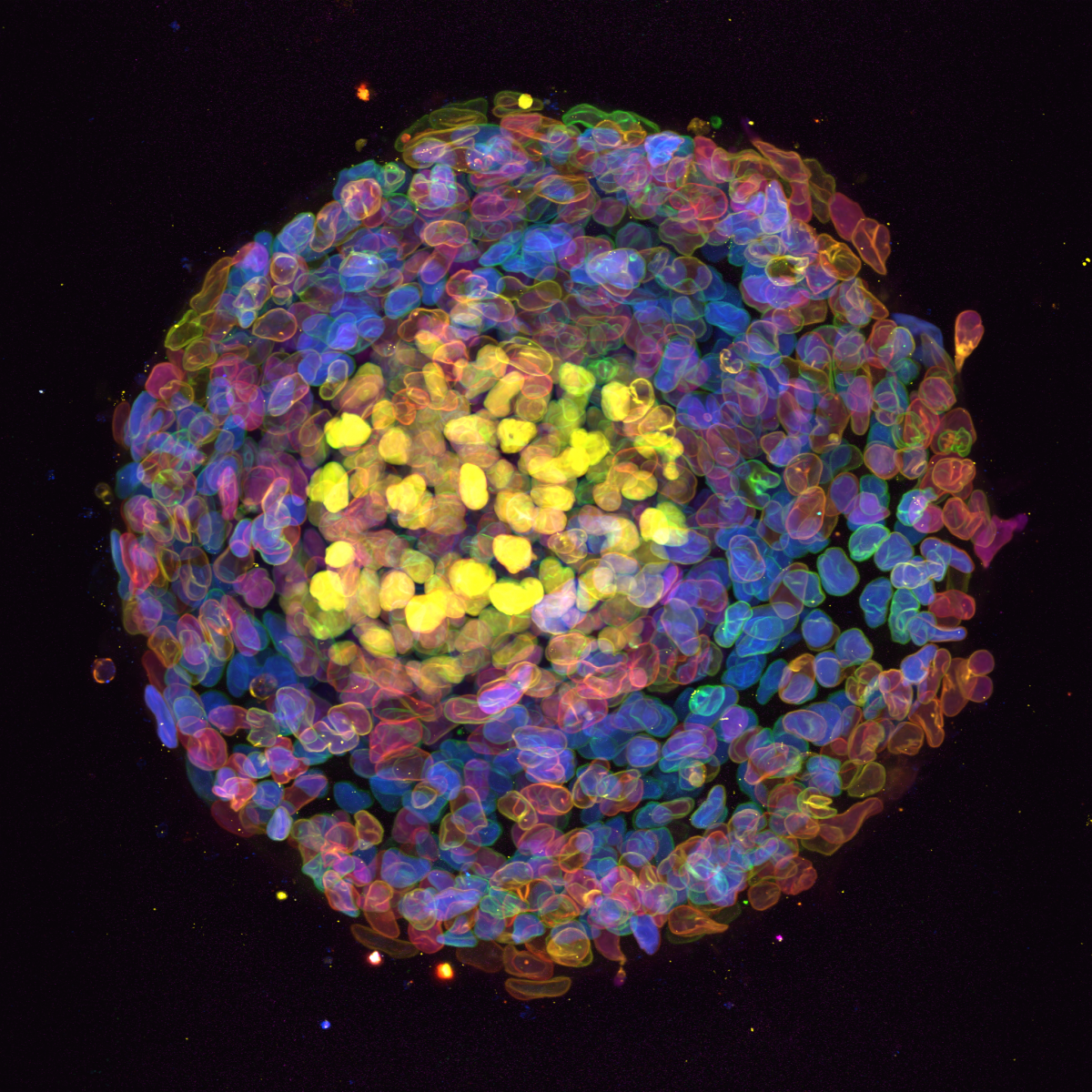
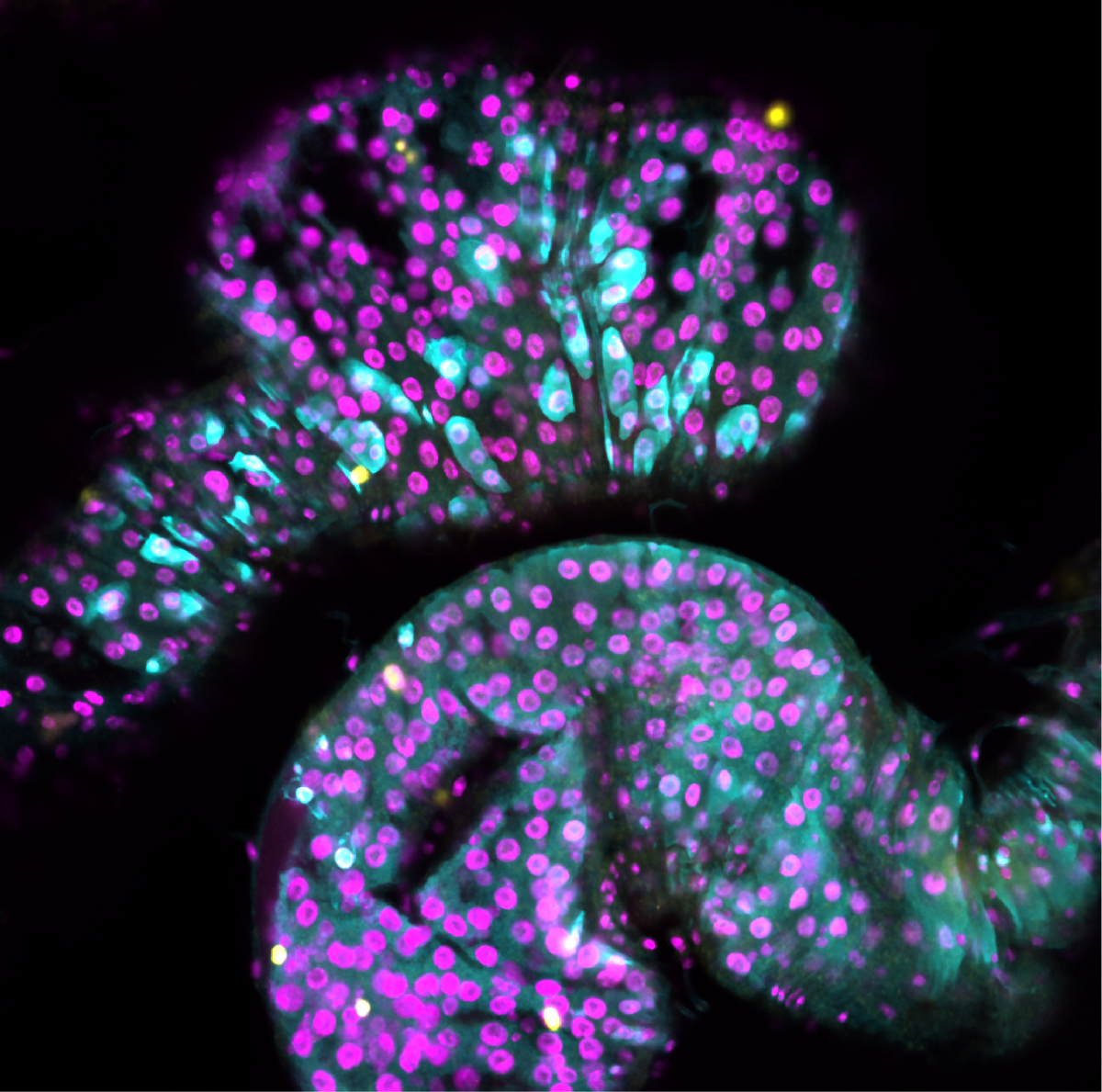
4. Organised organoid wreath
Matthieu Vermeren, University Of Edinburgh
Imaging Facility Manager, CRM Imaging Facility
Organoid imaged during a demo of the new Nikon AX confocal microscope in IGC, Edinburgh. This organoid was stained against three different transcription factor (Sox17, Sox2, TBra, coloured in blue, yellow and magenta) and a nuclear enveloppe marker (LMBR, shown as colour depth). The Blin lab (CRM, Edinburgh) looks at the effect of substrate geometry on organoid organisation. The substrate was patterned using PRIMO and seeded with stem cells. These self-organise to create this wreath-like structure.
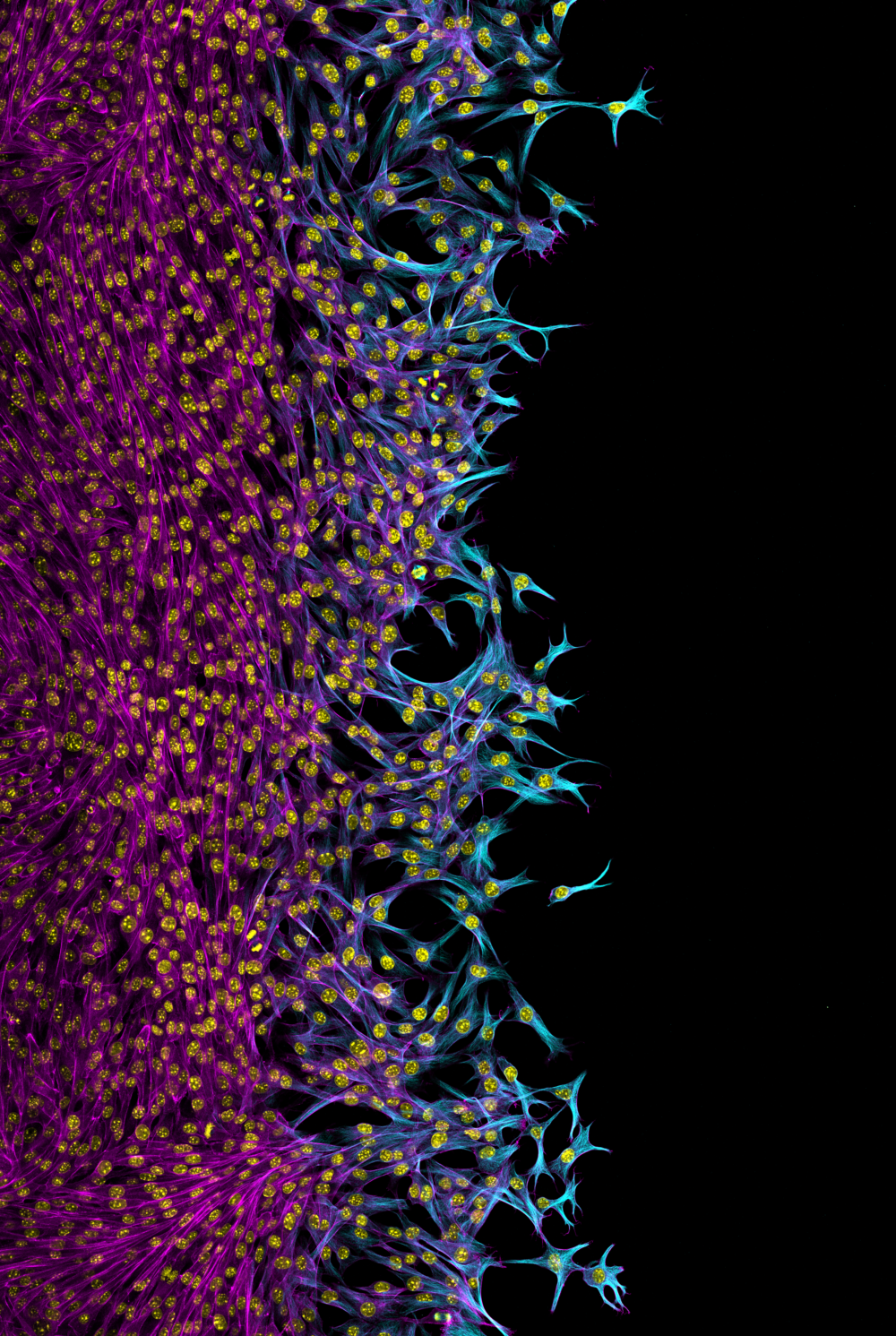
5. Invading cancer cells
Jamie Whitelaw, CRUK Beatson Institute
Post Doctoral researcher
NckAP1 KO B16-F1 cells migrating through matrigel in a circular invasion assay. Cells were fixed and stained using Phalloidin in magenta, Tubulin in cyan and DAPI in yellow. Z-stacked, tile scan images were taken on the Zeiss 880 AiryScan at 20X objective and displayed at a max projection. Note that for some unknown reason, the tubulin staining appears reduced in follower cells compared to the leader cells, while F-actin remains uniform throughout.
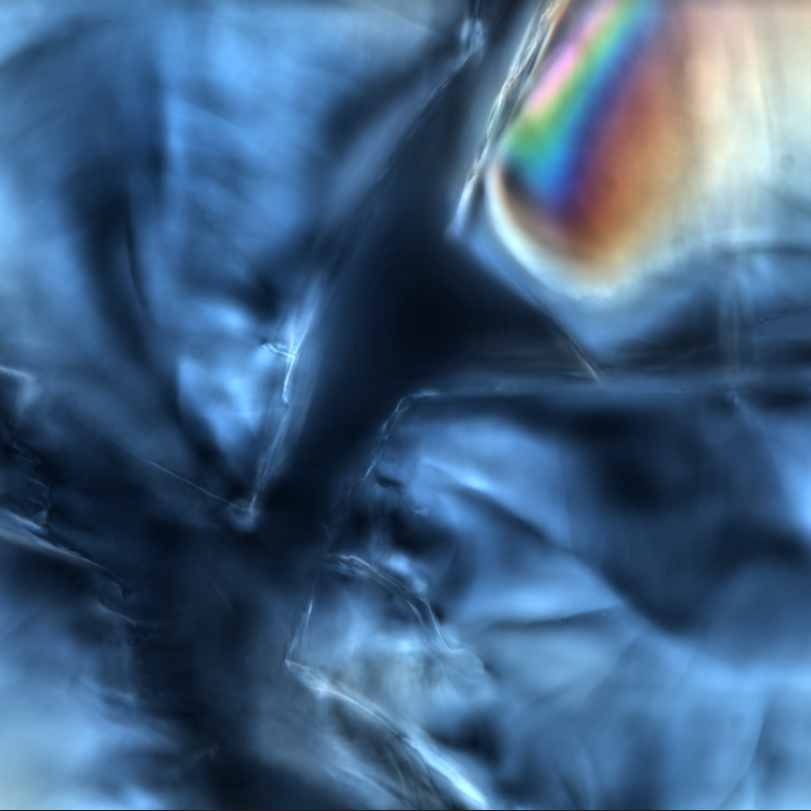
6. "Don't Stress Me Out"
Jordan Murray, University of Strathclyde
PhD Student
This image shows mechanical stress induced birefringence in Nafion polymer particles. These particles have been freeze fractured and imaged using differential interference contrast (DIC) microscopy, revealing areas of high mechanical stress in the form of beautiful spectral patterns.
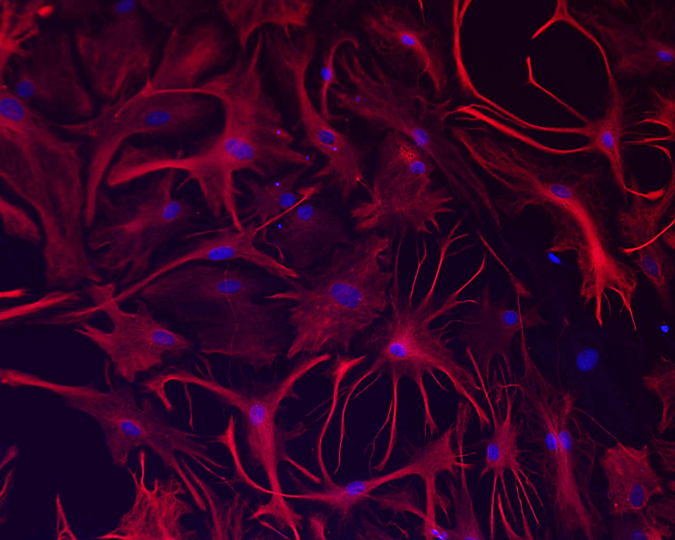
7. Astrocytes
Aya Abdallah, University of Aberdeen
PhD Student
Fluorescence staining of rat cortical astrocytes labelled intermediate filament protein with GFAP with fluorophore (Alexa-Flour 594 – Red) and nucleus (Hoechst-Blue). Image captured using 20x objective magnification of Nikon Ti-E fluorescence microscope.
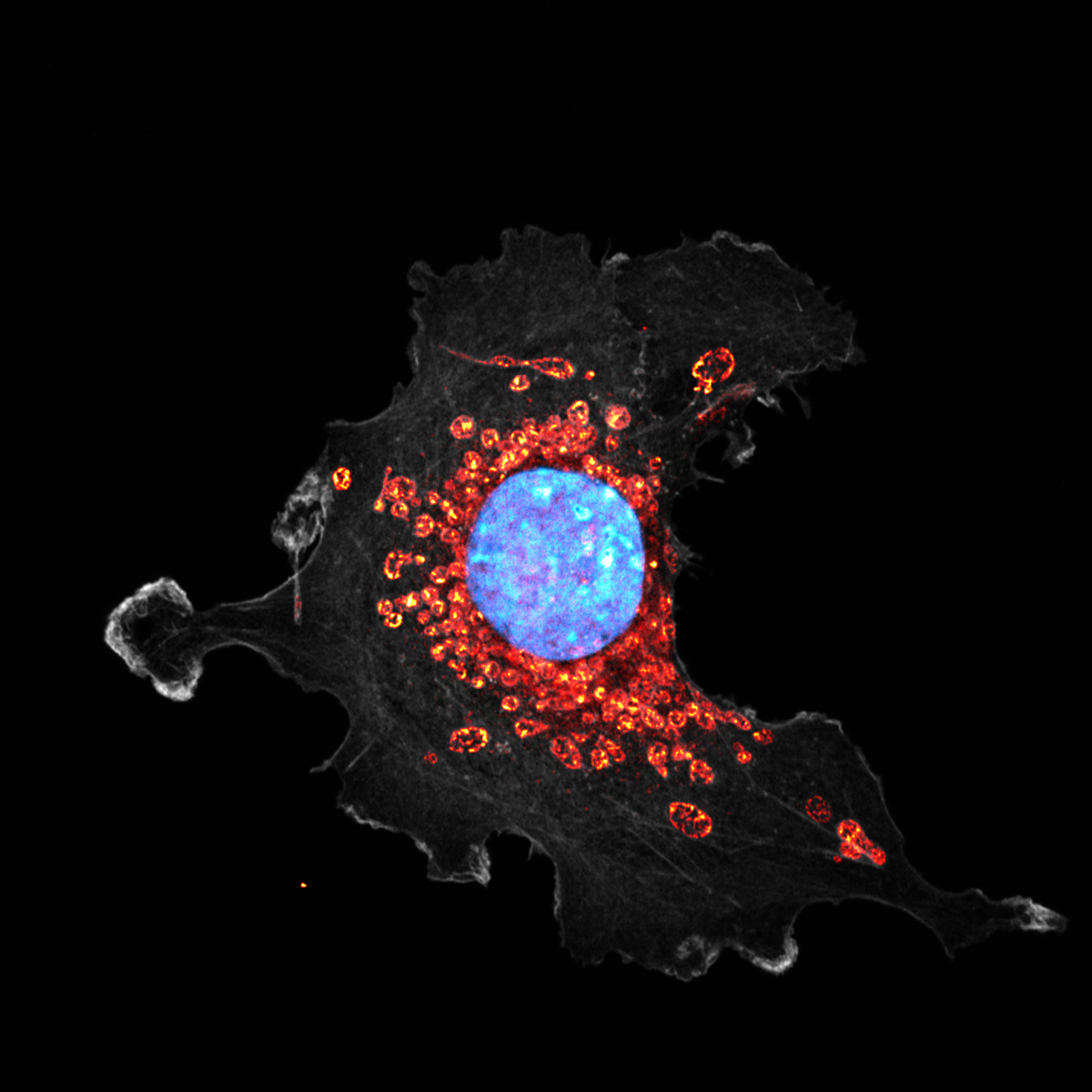
8. Mito At Work
Anh Le Hoang, CRUK Beatson Institute,
Scientific Officer
This image shows a fibroblast-like cell stained for the actin cytoskeleton (phalloidin, grey), nucleus (DAPI, blue) and mitochondria (MitoTracker Red CMXRos, orange). The cell was fixed with 4% paraformaldehyde and imaged using the Super-resolution microscopy with AiryScan Zeiss880 system at x630 magnification. The cell was depleted of a metabolic enzyme involved in cellular energy production. We hypothesised that this could affect the mitochondrial morphology and dynamic. This cell has particularly large mitochondria, which allows us to even visualise the cristae structures inside each mitochondrion. The image was taken at The Beatson Advanced Imaging Resource (BAIR).
9. Connecting the Heart
Ben Veerman, University of Strathclyde,
PhD Student
Connexins are gap-junctional proteins which are expressed within the heart and play a vital role to maintain a synchronous cardiac contraction. Connexin-43 (Cx43) is the most characterised gap-junction and is important for cell-to-cell communications between the different cardiac cell types, but mainly the cardiomyocytes. A rat heart was perfused by using the Langendorff technique for one hour prior cardiomyocyte isolation and staining. In the presented images, the Cx43 is localised at the lateral side of the isolated cardiomyocytes (green). F-actin is important to assemble thin filament proteins which are essential in contraction and relaxation, and it provides the scaffold of the cellular content via an F-actin-based cytoskeleton (magenta).
Cx43 was stained by using anti-Cx43 (C 6219 – Sigma Aldrich) and Alexa Fluor 488 goat anti-rabbit IgG (A11008 – Invitrogen) with an excitation of 488nm (green). Cellular F-actin was stained using rhodamine phalloidin with an excitation of 555nm (R415 – ThermoFisher) (magenta). The nucleus of the cells is counterstained with DAPI with an excitation of 405nm (D-9542 – Sigma, blue). The images were acquired by a Leica Confocal SP8 microscope using licensed Leica Application Suite X (LAS X) software using 63 x oil immersion objective (1024 x 1024 frame size). The scalebar is 25μm.
12. Keep Growing
Savvas Nikosia, CRUK Beatson Institute,
PhD Student
Pancreatic cancer cells proliferating on soft hydrogels as clusters. The cells were isolated from a KPC mouse model and cultured on soft hydrogels to recapitulate the tumour microenvironment. GFP positive cells (magenta), F-actin (yellow), Ki-67 marker to show proliferating cells (cyan) and Dapi (green). Maximum intensity projection of 3D z-stacks was used and imaged using Zeiss 880 LSM with Airyscan (63x objective). The microscope used is from the BAIR facilities (CRUK Beatson Institute)
13. E. coli biofilm or solar flares?
Beatrice Bottura, University of Strathclyde,
PhD Student
This image of a mature E. coli biofilm grown on an agar substrate shows the beauty and the complexity of internal biofilm architecture. A network of intra-colony channels spans the whole biofilm volume, and helps transport nutrients from the environment towards the centre of the biofilm. The irregular edge of the biofilm is reminiscent of solar flares.
Images were acquired in confocal laser scanning fluorescence microscopy using the Mesolens, our custom-made mesoscope with numerical aperture of 0.47 and 4x magnification, which allows to image a field of view of 6 mm x 6 mm with sub-micron lateral resolution. The image is a maximum intensity projection of a z-stack obtained over a total thickness of 207 μm. The bacterial cells forming the biofilm express GFP fluorescence, which is excited using a 488 nm laser and detected with a PMT.
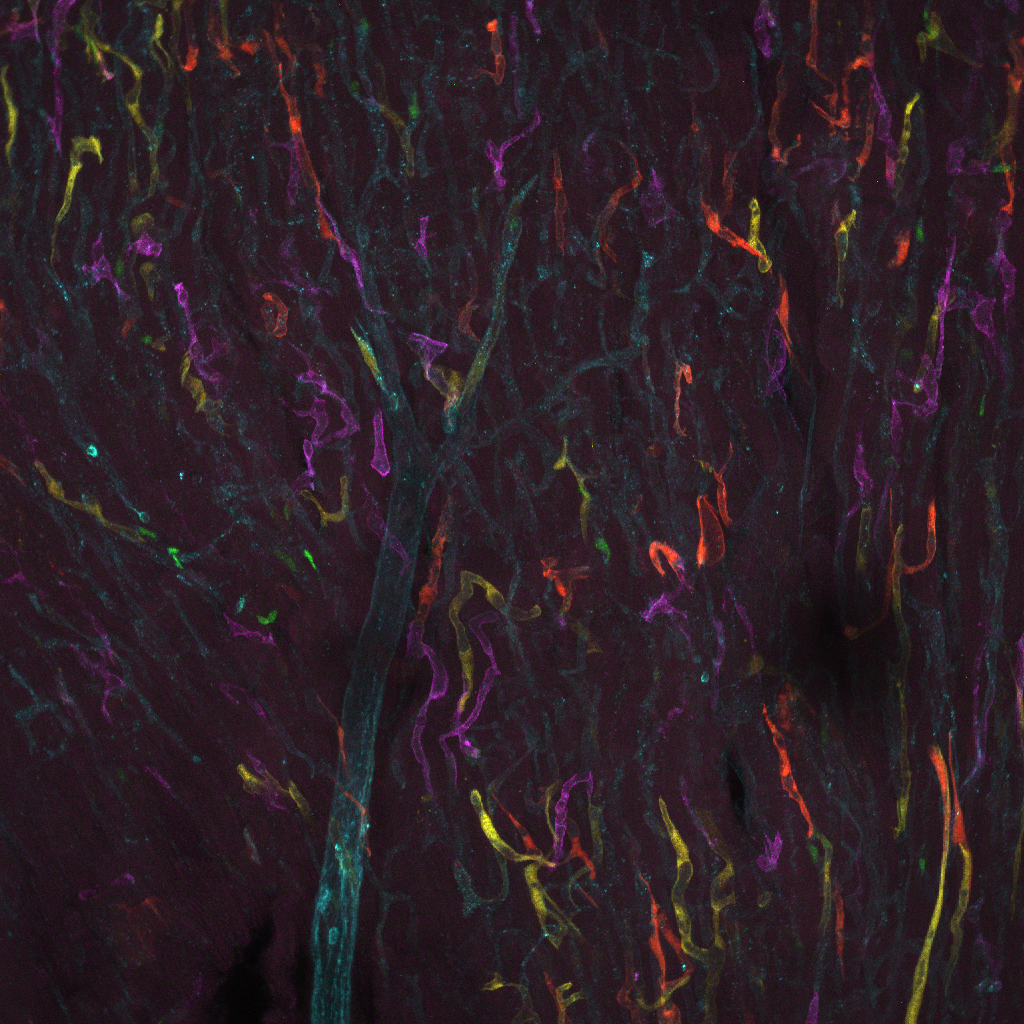
14. The inner fire-workings of the heart
Bronwyn Berkeley, University of Edinburgh,
PhD Student
This image shows the blood vessel network in a slice of heart taken from a mouse. Located centrally in the image we can see a larger blood vessel that branches out. The multi-colored confetti we can see in the image are in fact blood vessels! The endothelial cells, that line the inside of all blood vessels, in these special “Confetti” mice are marked with colored fluorescent proteins.
15. Breaking Bad
Yvonne Odey, University of Edinburgh,
Post Doctoral Researcher
An immature human oocyte (red, rhodamine-phalloidin (TRITC), F-actin) surrounded by cumulus cells (green, Alexa Fluor Plus 488, tubulin) after in vitro maturation (IVM). IVM supports the resumption of meiosis (which has been arrested at Prophase I when the oocytes were formed before birth) through the first division to Metaphase II and the formation of the first polar body, which is discarded from the oocyte. IVM can be used as an option for fertility preservation.
This oocyte was obtained from a young transman whose ovaries were removed at the time of gender reassignment surgery. Whilst maturation to Metaphase II appeared to have occurred, confocal examination shows a disorganised spindle (green, tubulin located left centre of the oocyte) and abnormal migration and removal of the polar body. DAPI (blue) stains the DNA of all the cells. This image highlights that the quality of oocytes following IVM may be compromised.
Image captured at the IMPACT imaging facility (CDBS) at the University of Edinburgh, using a 60X Plan Apo VC/ NA 1.4 OIL objective on a Nikon A1R confocal microscope.
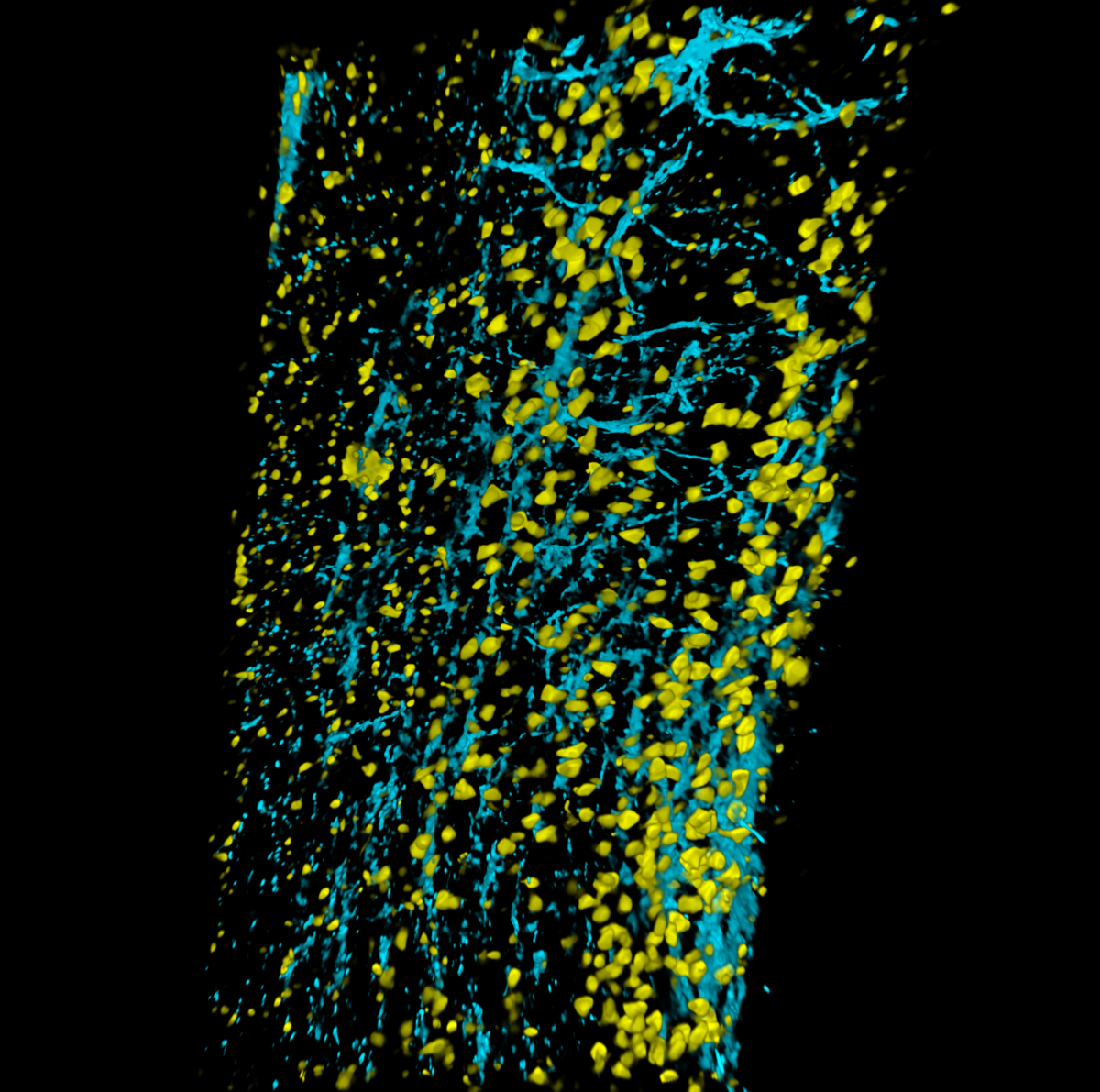
16. See Through Spinal Cord
Karthik Krishnamurthy, Thomas Jefferson University,
Post Doctoral Fellow
Immunolabeling-enabled three-dimensional imaging of solvent-cleared organs (iDISCO) of 4-month-old mouse spinal cord. Astrocytes are labeled with Glial fibrillary acidic protein (blue) and neurons are labeled with NeuN (yellow). Video represents Z stack imaging through the whole spinal cord using Nikon A1R confocal microscope.
17. Human organoid synaptic connections
Karthik Krishnamurthy, Thomas Jefferson University,
Post Doctoral Fellow
Human cerebral brain organoids grown for 10 weeks invitro and cleared with iDISCO. Cleared samples are labeled with synaptic vesicle protein SV2 (red), neuronal marker MAP2 (Yellow), and nuclear marker DAPI (blue). Image captured using Nikon A1R confocal microscope 60x.




Get in touch with the Scottish Microscopy Society!
Feel free to contact us or join our mailing list using the contact form below.